Paperless Forms 2.0 – Digitizing your Test Data
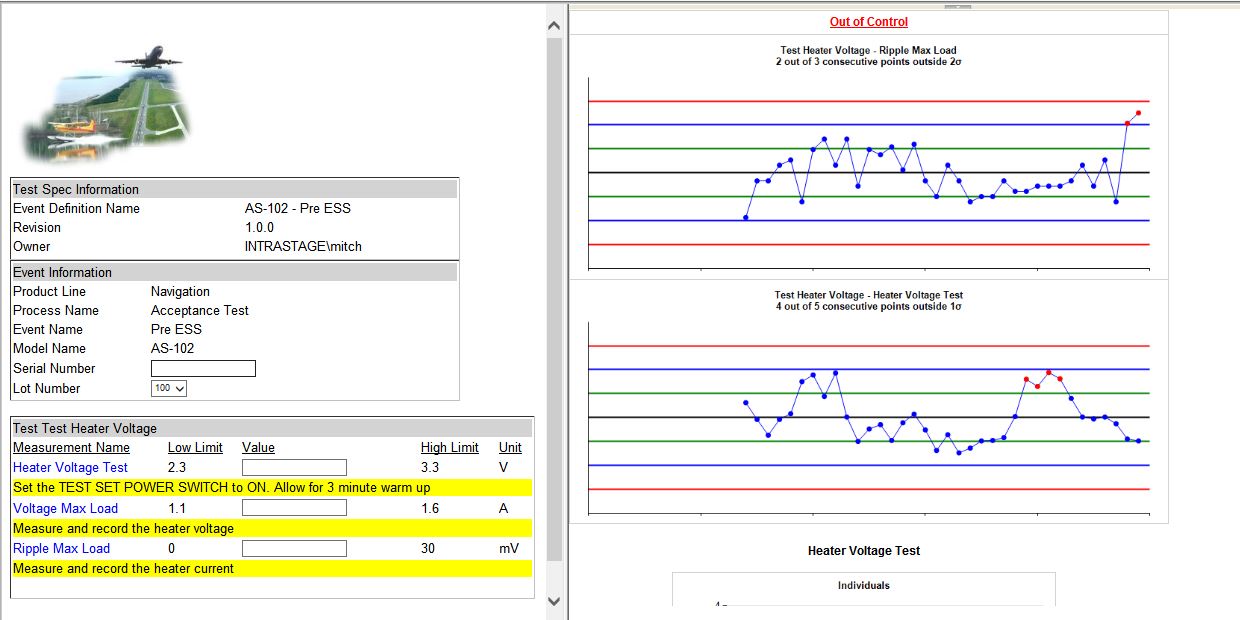
- Real-Time SPC on Test Data
- Web Based Forms for Test Data Collection
- Advance Reporting Capabilities
- Dashboard views of SPC Trends from anywhere in the World
Recall and Return events on Medical Device and Aerospace products can have serious outcomes where the FDA, your customers or other authorities quickly want to understand and root cause failures. These events have caused the industry to create rules around Compliance and Traceability on the Test Data.
In many manufacturing environments today, much of the Test Data is still recorded in Paper based processes. Paper based Test Data potentially means inaccurate recording, manual processes to retrieve that data and difficulty in aggregating the data to get an overall view of Quality. IntraStage Paperless forms solution was created to solve the massive amounts of Paper based Test Data that is currently being taken in Manufacturing environments around the world and give additional advantages:
- Quality engineers and inspectors are able to measure, manage, and report on quality activities as they happen, in real-time without straining resources.
- Seamless communication between Design and the Manufacturing teams. This means that you can quickly investigate and resolve issues faster
- Eliminate inaccurate spreadsheets, manual processes, and error-prone paper trails that come with hard-copy documentation.