Managing Supplier Quality with Remote Data Monitoring and Alerts
Managing Supplier Quality with Remote Data Monitoring and Alerts
Summary
This IntraStage Case Study describes the benefits that were realized by Teradyne’s application and use of the real-time IntraStage Supplier Quality System. Benefits of implementation of the IntraStage solution included faster time to manufacturing, better quality, and lower costs.
Background
When producing highly complex test equipment, Teradyne is no stranger to cost, time and urgency. As a world leader in the automatic test equipment (ATE) market, Teradyne is a leader in the application of thorough, comprehensive test processes and quality control. As part of its commitment to its customers to produce an ATE tester that is free of defects, reliable, and capable, Teradyne’s focus on quality is not only dependent on quality from the manufacturing floor, but also from supplier-sourced components.
Bryan Johnson, a supplier engineering manager at Teradyne, foresaw a problem with their current supplier management: “We proactively worked with suppliers to qualify parts and establish robust control plans. But, in addition to normal process audits, we needed to assure the control plans at our suppliers were being executed based on real time data collection and analysis. We were also concerned that processes for our most challenging parts could drift over time, impacting yields and the ability of suppliers to ship the quantity of parts we needed on time. We needed Statistical Process Control (SPC) deployed for a global supply base that often-lacked knowledge and experience with SPC methodologies and needed a data archive for our most challenging and important component specifications.”
Requirements
Instead of depending on suppliers to both learn and develop skills in SPC, Bryan and his team started evaluating options for performing what he calls ‘remote data monitoring and alerts’: making sure that the suppliers are capturing critical parametric performance metrics automatically before their product ships out to Teradyne. ‘It was critical that we have a blended model of data input: both manual data entry, and to support existing file outputs by those suppliers with minimal disruption to their processes and productivity. We also wanted to make sure that suppliers could benefit from the system and gather the data that would help them to improve their processes, yield and quality.’
With multiple suppliers, including some suppliers making the same component, security and supplier access to the correct information were also critical. Data from these processes can remain in silos, which creates obstacles for Teradyne engineers to access, analyze, and use to improve testing processes and product design. Therefore, the Teradyne engineers would have to maintain a complete view of all suppliers’ data and performance. The engineers’ goals were to:
– Minimize quality-based supply chain disruptions to ensure Just In Time (JIT) manufacturing
– Ensure early visibility of supplier quality issues
– Reduce customer recalls and quality issues
– Maintain yields of our most challenging parts
Implementation
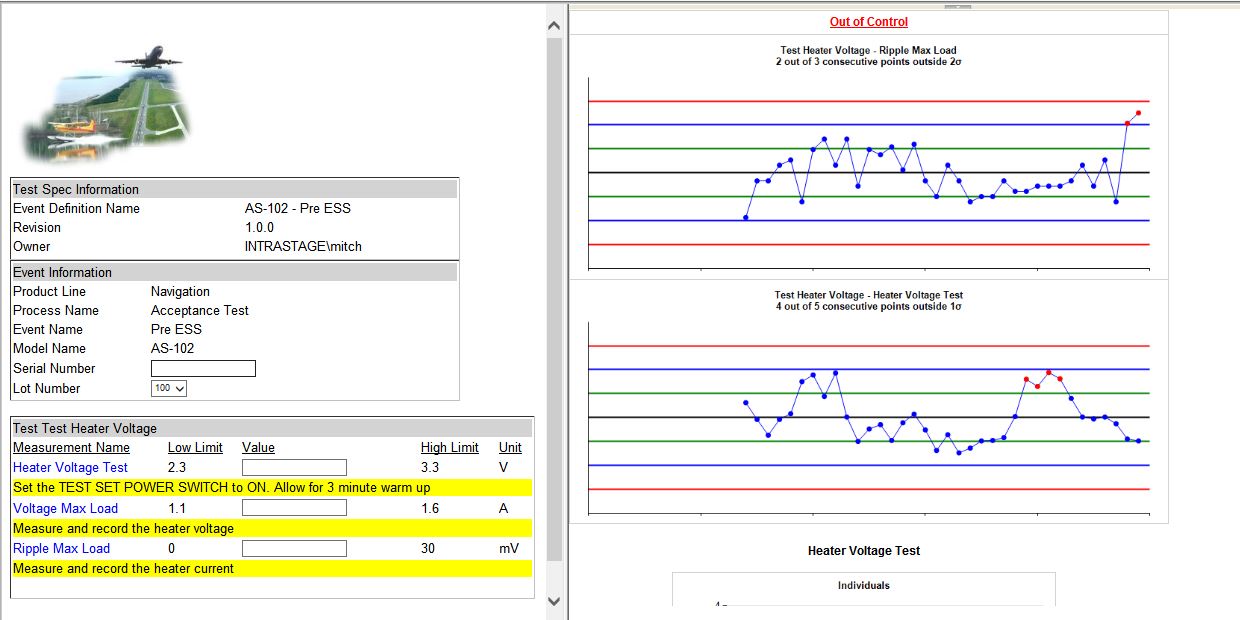
Using a blend of IntraStage’s Paperless Manufacturing product and customer adapters allowed Teradyne to incorporate data from multiple sources. The Paperless Manufacturing forms were customizable via a GUI, which allowed Teradyne engineers to self-serve and develop new forms quickly, and to edit the forms to capture richer metadata from the suppliers. The custom adapters converted source data into the IntraStage cloud-based database, allowing real-time visibility of the test data by both Teradyne and the suppliers. By receiving and being alerted on this data in near real-time, Teradyne and their suppliers could take action on potentially negative emerging trends.
Analysis
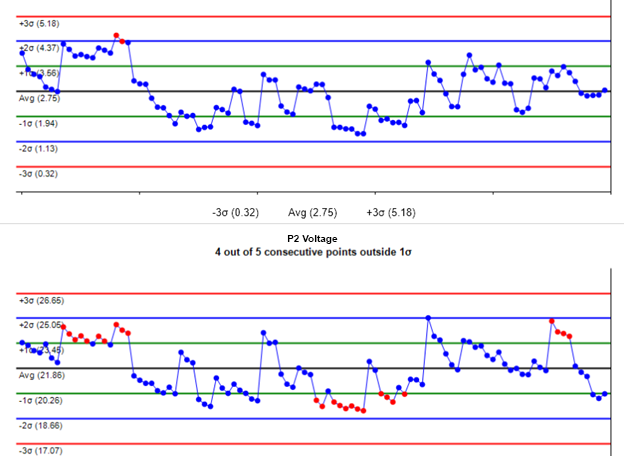
A key output of the data capture was the implementation of Western Electric Rules and Statistical Process Control analytics on the source data. Bryan Johnson’s team was not satisfied if the data were merely passing; they wanted to see if data were starting to develop negative capability indexes based on Cpk and Cp. By keeping an eye on SPC tables giving every parametric measurements’ Cp and Cpk, a quick visual check could easily identify performance. Drilldown into each parametric measurement on Xbar-R and characterization charts could quickly identify useful variables responsible for performance degradation. By identifying emerging trends, corrective actions were taken prior to potentially bad components even being shipped to Teradyne.
Lessons learned
By keeping critical supplier data in an off the shelf IntraStage database, with near real-time alerts and notifications, Teradyne has been able to better work with suppliers to maintain tight quality and time to market. Teradyne is maintaining and growing market share by maintaining brand quality and customer satisfaction with it world-class testing equipment. IntraStage’s BlackBelt manufacturing system gave Teradyne the tools it needed to not only understand where its supplier parts needed additional testing and attention, but detect emerging trends in real time to avoid issues in the first place.
For more information, contact sales@intrastage.com